The essence of lithium battery rapid charge and discharge is that lithium ions can be rapidly embedded between positive and negative materials. Battery material properties, process design, and charge and discharge regimes all have an impact on high current charging performance.
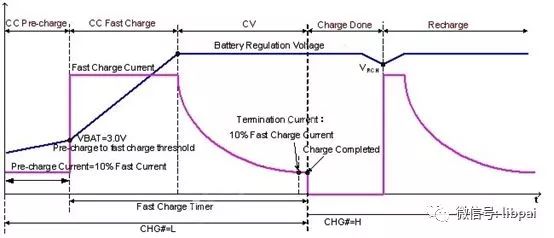
Use this figure to illustrate the process of charging the battery. The abscissa is time and the ordinate is voltage. Lithium batteries have a small current precharging process at the beginning of charging, that is, CC Pre-charge. The purpose is to stabilize the positive and negative materials. After that, the battery state can be adjusted to charge a large current, that is, CC Fast Charge.
Finally, enter constant voltage charging mode (CV). For the lithium battery, the system starts the constant voltage charging mode after the voltage reaches 4.2V, and the charging current gradually decreases. Finally, the charging is completed after the voltage is less than a certain value.
In the whole process, there are different standard charging currents for different batteries, such as 0.1C-0.5C for 3C battery standards, and 1C for high-power batteries. Selecting a lower charging current also takes into account the safety of the battery. Therefore, the fast charge, which is usually said, means that the charge current is higher than the standard charge current by a factor of several to several tens of times.
Some people say that charging a lithium battery is like pouring beer. It's fast and it's full of beer, but it has a lot of bubbles. Slow down, slow, but the beer is very real. Fast charging saves the charging time, but also has a greater damage to the battery itself.
Due to the presence of polarization in the battery, the maximum charge current that it can accept decreases with increasing charge and discharge cycles. When the charge is continued and the charge current is large, the ion concentration at the electrode increases and the polarization increases. The terminal voltage cannot directly and linearly correspond to the charge/energy charged. At the same time, high-current charging, the increase of internal resistance will cause Joule heating effect to increase (Q = I2Rt), and bring about a series of problems such as the reaction of the electrolyte decomposition, gas production and other issues, a sudden increase in the risk factor, the safety of the battery Impact, the life of non-powered batteries will inevitably be greatly reduced.
01 positive electrode material
The process of rapid charge of a lithium battery is the process of Li+ rapid migration and insertion into the negative electrode in the positive electrode material. The particle size of the positive electrode material can influence the response time and the diffusion path of ions in the electrochemical process of the battery. According to studies, as the grain size of the material decreases, the diffusion coefficient of lithium ions increases. However, as the particle size of the material decreases, severe particle agglomeration occurs in the pulping during production, resulting in uneven dispersion. At the same time, the nanoparticles reduce the compaction density of the pole piece and contact with the electrolyte during charge and discharge. Area increases side effects and affects battery performance.
The more reliable method is to modify and modify the positive electrode material. For example, LFP itself is not very conductive, and its surface can be coated with carbon material or other materials to improve its conductivity, which can improve the rapid charging of the battery. performance.
02 anode material
Lithium-ion battery fast charge means that lithium ions quickly come out and "sweep" to the negative electrode. At this time, it is necessary for the negative electrode material to have a rapid lithium insertion capability. Anode materials for fast charging of lithium batteries include carbon materials, lithium titanate, and other novel materials.
For carbon materials, due to the lithium insertion potential and the lithium deposition potential, lithium ions are preferentially embedded into graphite in the case of conventional charging, but under fast charging or low temperature conditions, lithium ions may precipitate on the surface to form dendritic lithium. . Dendritic lithium punctures SEI, causing secondary loss of Li+ and reducing battery capacity. When the lithium metal reaches a certain amount, it will grow from the negative electrode to the separator, causing the battery to be short-circuited.
For LTO, it belongs to the "zero strain" oxygen-containing anode material. It does not generate SEI when the battery is working, and it has stronger binding ability to lithium ions, which can meet the requirements of fast charge and fast discharge. At the same time, because the SEI cannot be formed, the negative electrode material will be in direct contact with the electrolyte and promote the occurrence of side reactions. The problem of LTO battery gas production cannot be solved, and can only be mitigated by surface modification.
03 Electrode solution
As mentioned earlier, due to the inconsistency of the lithium ion migration rate and the electron transfer rate in the fast charge process, the battery will have a large polarization. So in order to minimize the adverse reactions caused by battery polarization, we need to develop the electrolyte from the following three points: 1. Electrolyte salt with high dissociation degree; 2. Compound with solvent-lower viscosity; 3. Interface control - Membrane impedance Lower.
04 Production Process and Fast Charge
Previously, the requirements and influences of fast charge on three key materials, namely positive and negative materials and electrode fluids, were analyzed. The following is a comparatively large process design. The manufacturing process parameters of the battery directly affect the migration resistance of lithium ions in various parts of the battery before and after the activation of the battery, so the battery preparation process parameters have an important influence on the performance of the lithium-ion battery.
(1) Slurry
For the properties of the slurry, on the one hand, the uniform dispersion of the conductive agent is maintained. Since the conductive agent is uniformly distributed between the active material particles, a more uniform conductive network can be formed between the active materials and between the active material and the current collector, which has the function of collecting micro currents, reducing the contact resistance, and increasing the electron transfer rate. . On the other hand, the excessive dispersion of the conductive agent is prevented. In the charge-discharge process, the crystal structure of the positive and negative electrode materials may change, which may cause the peeling off of the conductive agent, which may cause the internal resistance of the battery to rise and affect the performance.
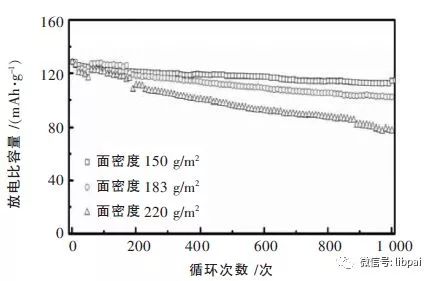
(2) Polar surface density
In theory, the rate-type battery and high-capacity battery can not have both. When the surface polarities of the positive and negative polar plates are low, the diffusion rate of lithium ions can be increased, and the ion and electron transfer resistance can be reduced. The lower the areal density, the thinner the pole piece, and the smaller the change in the structure of the pole piece caused by constant insertion and extraction of lithium ions during charge and discharge.
However, when the areal density is too low, the battery energy density is reduced and the cost is increased. Therefore, it is necessary to comprehensively consider the areal density. The following figure is an example of a lithium cobalt oxide battery 6C charging 1C discharge, you can see:
(3) Pole coating consistency
Before a friend asked, does the inconsistent polar density affect the battery? Here, by the way, for the fast charge performance, it is mainly the consistency of the negative pole piece. If the negative surface density is not uniform, there will be a large difference in the internal porosity of the active material after rolling. The difference in porosity can cause differences in the internal current distribution, affect the formation and performance of SEI in the cell formation stage, and ultimately affect the fast charge performance of the battery.
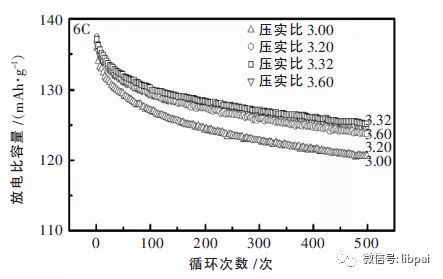
(4) Compaction density
Why do pole pieces compact? One is to increase the specific energy of the battery, and the other is to improve the battery performance. Different electrode materials, the best compaction density is also different. To increase the compaction density, the smaller the porosity of the electrode piece, the more closely the particles are connected, and the smaller the thickness of the piece piece is at the same areal density, so the lithium ion migration path can be reduced.
When the compaction density is too large, the electrolyte infiltration effect is not good, and the structure of the material and the distribution of the conductive agent may be damaged, and the winding problem may occur later. The same lithium cobalt oxide battery 6C charge 1C discharge, compaction density of discharge specific capacity as shown below:
05 into aging and other
For carbon negative batteries, chemical-aging is a key process for lithium batteries. This process affects the quality of SEI. SEI thickness or structural instability, will affect the battery's fast charge capacity and cycle life.
In addition to the above several important factors, the battery production, charge and discharge systems will have a greater impact on the performance of lithium batteries. As the use of time is extended, the battery charge rate should be appropriately reduced, otherwise the polarization will be exacerbated.
Conclusion
The essence of lithium battery rapid charge and discharge is that lithium ions can be rapidly embedded between positive and negative materials. Battery material properties, process design, and charge and discharge regimes all have an impact on high current charging performance. The structural stability of the positive and negative materials facilitates the collapse of the structure during the rapid delithiation process. Lithium ions diffuse faster in the material to withstand large current charging. Since the ion migration rate and the electron transfer rate do not match, polarization occurs during charge and discharge. It is necessary to minimize the polarization and prevent the precipitation of lithium metal and reduce the influence of the capacity on lifetime.
A separate slip ring is a device that allows two or more rotating electrical shafts to be connected without having to pass the electricity through the shafts' bearings. This is often used in situations where there is a need to power something externally while the shaft is still turning. For example, a machine might have a drive shaft that powers it while also having a separate output shaft that needs to turn at a different speed. By using a separate slip ring, these two shafts can be powered without any interference.
Slip Ring Shaf is a key component of a slip ring. It is the shaft on which the rotary electrical contacts are mounted. The shafts must be strong enough to support the weight of the contacts and must be able to turn freely. There are many factors to consider when selecting a shaft material, including strength, corrosion resistance, and operating temperature.
Oubaibo is a professional separate slip ring manufacturer located in China. They offer a wide range of slip ring shaft made from different materials, including carbon steel, stainless steel, and brass. Their products are made to meet the highest quality standards and are backed by a 100% satisfaction guarantee.
Separate Slip Ring,Slip Ring Shaft,Slip Ring 4 Wire,Slip Ring For Motor
Dongguan Oubaibo Technology Co., Ltd. , https://www.sliprob.com
