The theoretical specific energy of aluminum-air fuel cells can reach 8100Wh/kg, which has the advantages of low cost, high specific energy density and high specific power density. As a special fuel cell, aluminum-air batteries have great commercial potential in applications such as military, civil, and underwater power systems, telecommunications system backup power sources, and portable power supplies.
Metal Air Battery Overview
Lithium-ion batteries have higher specific energy, and are relatively mature and already large-scale commercial secondary batteries. However, in recent years, lithium-ion batteries have been difficult to meet in the face of tremendous development in the fields of mobile electronic devices and electric vehicles. Its large capacity needs, especially for power battery systems that are highly dependent on energy. Therefore, metal air batteries having a capacity several times larger than that of lithium ion batteries have emerged, such as zinc air batteries, aluminum air batteries, magnesium air batteries, and lithium air batteries.
Since the positive active material of such a battery is mainly derived from oxygen in the air, the theoretical amount of the positive active material is infinite, so the theoretical capacity of the battery mainly depends on the amount of the negative metal, and such a battery has a larger specific capacity.
Among them, the theoretical specific energy of aluminum-air fuel cells can reach 8100Wh/kg, which has the advantages of low cost, high specific energy density and high specific power density. As a special fuel cell, aluminum-air batteries have great commercial potential in applications such as military, civil, and underwater power systems, telecommunications system backup power sources, and portable power supplies.
Aluminum air battery structure and principle
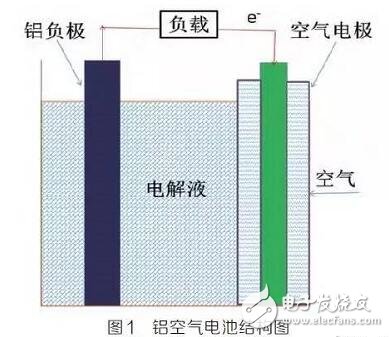
Figure 1 is a schematic diagram of the structure of an aluminum air battery
From the analysis of existing research results and battery characteristics, aluminum air batteries have the following characteristics:
(1) Higher specific energy. Aluminum air battery is a new type of high-energy battery. The theoretical specific energy can reach 8100Wh/kg. The current research and development products can reach 300-400 Wh/kg, which is much higher than the specific energy of various types of batteries.
(2) The specific power is medium. Since the working potential of the air electrode is far from its thermodynamic equilibrium potential, its exchange current density is very small, and the battery is highly polarized when discharged, resulting in a specific power of the battery of only 50-200 W/kg.
(3) Long service life. The aluminum electrode can be replaced continuously, so the life of the aluminum air battery depends on the working life of the air electrode.
(4) Non-toxic, no harmful gases. The electrochemical reaction of the battery consumes aluminum, oxygen and water to form Al2O3·nH2O, which can be used for drying adsorbents and catalyst carriers, grinding and polishing abrasives, ceramics and excellent precipitants for sewage treatment.
(5) Strong adaptability. The battery structure and raw materials used can be changed according to the practical environment and requirements, and have strong adaptability.
(6) Battery anode material Aluminum is cheap and easy to obtain. Compared with other metals, the price of metal aluminum is relatively low, and the manufacturing process of metal anode is relatively simple.
Aluminum anode (negative electrode)
Aluminum (Al) is an ideal electrode material. The theoretical energy density of aluminum metal is 8.2 W·h/g. Among common metals, it is only 13.3 W·h/g below lithium, and the electrode potential is negative. Light metal battery material with the highest mass ratio energy other than metallic lithium. The mass ratio energy of the aluminum air battery can actually reach 450Wh/kg, and the specific power reaches 50~200 W/kg. It has the advantages of high theoretical capacity, low consumption rate, light weight, negative potential, abundant resources and easy processing.
However, since aluminum is a very active amphoteric metal, the development of aluminum anodes is currently affected by the following problems.
(1) There is a passivation film on the aluminum surface, which affects the electrochemical activity of aluminum.
(2) Aluminum is an amphoteric metal element, which determines that it is easy to decompose hydrogen corrosion in a strong alkaline environment, affecting the electrode potential, and the product floats in the electrolyte to affect the entire electrochemical reaction.
(3) The unique semi-open system of the air battery makes the air electrode susceptible to external humidity, causing “submersion†or “drying†of the aluminum anode, or even “clinding†or “leakageâ€, thus the whole air The structure of the battery caused damage. In order to solve the above problems, domestic and foreign scholars have studied from the following three aspects:
1. Aluminum anode alloying
Industrial grade aluminum (99.0%) contains more impurities, such as iron (0.5%), silicon, copper, manganese, magnesium and zinc, which will increase the hydrogen evolution corrosion of aluminum at the interface, especially iron will form a part with aluminum. The primary battery causes the electrochemical corrosion to multiply. An alloy component which can improve chemical activity and improve corrosion resistance can be added to aluminum.
The conditions that need to be met for the alloying of aluminum alloys are as follows: 1 the melting point of the alloying elements is lower than that of the metal Al; 2 the solid saturation is higher in Al; 3 the electrochemical activity is higher than that of Al; 4 is more soluble in the electrolyte High; 5 has a high hydrogen evolution overpotential. In addition, by processing the anode metal into an ultrafine grain material, the anode efficiency can be further improved.
2. Add a slow release agent to the electrolyte.
Because of the cost of anode alloying, people often choose to add some slow release agent to the electrolyte to ensure the performance of the aluminum air battery. Some carboxylic acid, amine, and amino acid sustained-release agents and their inhibition efficiency against aluminum corrosion are shown in Table 1:
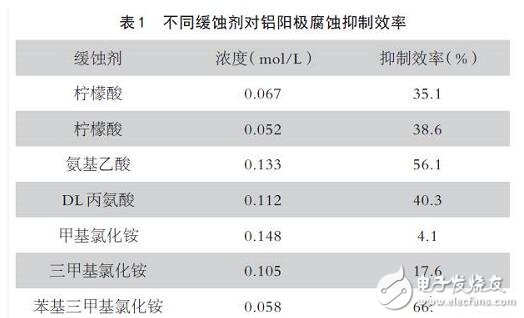
The researchers used natural materials as inhibitors of metal aluminum corrosion. Experiments have shown that organic amines, pyrrole and the like have a significant inhibitory effect on aluminum corrosion. By adding organic matter and water-soluble compounds to the strong alkali electrolyte, the electrochemical behavior of the aluminum metal anode is studied to reduce the corrosion rate of aluminum, thereby improving the performance of the aluminum air battery.
3, heat treatment process
Heat treatment affects the properties of the alloy by changing the distribution of trace elements in the aluminum alloy and the microstructure of the alloy surface, which belongs to the research field of technology. The best heat treatment process can be found by suitable orthogonal experiments.
Electrolyte
The electrolyte of the aluminum air battery is mostly a neutral salt solution or a strong alkaline solution. When a neutral electrolyte is used, the anode self-corrosion is small, but the surface of the aluminum anode is heavily passivated, the working voltage is lowered, the power and current of the battery are difficult to increase, and the voltage hysteresis is caused, and the product aluminum hydroxide colloid is also settled and blocked. Electrolyte, so this type of battery can only be used as a low-power power output device.
When a strong alkaline electrolyte is used, the passivation of aluminum is reduced, and the alkali solution can absorb a certain amount of the reaction product aluminum hydroxide. The performance of the battery is relatively good, but aluminum is an amphoteric metal, which occurs in a strong alkaline environment. Strong hydrogen evolution corrosion, release a large amount of hydrogen, reduce the output power of the battery and anode utilization, more serious at high current density. If it is simply to solve the above problems, you can choose to periodically replace the electrolyte and add an additive to the electrolyte that activates the surface of the aluminum anode and aluminothermic corrosion to solve the appeal problem.
Air electrode (positive electrode)
The cathode is the reaction site of O2, which has gas permeability, electrical conductivity, water resistance, corrosion resistance and catalysis. It is also often called air electrode. The air electrode generally consists of a porous catalyst layer, a conductive current collector and a waterproof gas permeable layer. The porous catalyst layer is the main site for the reduction of oxygen, where the diffused oxygen, oxygen reduction catalyst and thin electrolyte are formed at the interface. The three-phase interface electrochemical active site; the conductive current collector mainly functions as a conductive and mechanical support; the waterproof gas permeable layer has a porous porous water structure, which not only provides the gas required for the reaction to the catalytic layer, but also prevents the electrolyte from diffusing the gas. The channel is submerged.
The catalytic layer is the most critical part of the air electrode and plays a decisive role in its electrochemical performance. The performance of the aluminum air battery depends largely on the cathode catalyst chosen. The performance of the air electrode can directly affect the balance of the electrode reaction. Therefore, improving the performance can improve the utilization of the anode of the aluminum air battery to some extent and inhibit the self-corrosion of the anode aluminum.
Catalysts for commonly used catalysts for aluminum air batteries are as follows:
(1) Precious metal catalyst. Platinum and silver are commonly used, and their catalytic activity is relatively high in performance, but the rate is not high because of the relatively high price and shortage of resources.
(2) A metal macrocyclic compound catalyst. Organometallic macrocycles have good catalytic activity for oxygen reduction, especially when they are adsorbed on large surface area carbon. And their activity and stability can be significantly improved by heat treatment. Therefore, it is expected to replace the noble metal oxygen reduction catalyst. Common methods for synthesizing metal macrocyclic compounds include thermal decomposition and precursor preparation. However, since the heat treatment process of the thermal decomposition method causes the reaction of the metal macrocyclic compound with the carbon matrix, the catalyst prepared by the precursor method has poor activity, so there are certain problems in the application.
(3) Perovskite-type oxide catalyst. Perovskite-type oxides have high catalytic activity for reduction and precipitation of oxygen, and are inexpensive, so they have broad application prospects in aluminum air batteries and fuel cells. The current research on perovskite oxygen electrode catalysts mainly focuses on improving the preparation method and finding new substitution elements to improve the catalytic performance. The amorphous precursor method, especially the malic acid precursor method, can prepare perovskite oxides with fine crystal grains and large specific surface area, thereby greatly improving their catalytic activity, and is currently a better preparation of perovskite oxide. Methods.
(4) Cheap catalysts. The most important representative is manganese dioxide catalyst. Its biggest advantage lies in the abundant raw materials and low cost. It can be widely used in water-based or non-aqueous electrolyte batteries, but the single manganese dioxide electrocatalytic activity has certain limitations, so people are here. Research has never stopped.
(5) AB2O4 spinel type oxide catalyst. The crystal lattice of the spinel is a face-centered cubic. There are 32 closely packed 02-ions in the unit cell, 64 tetrahedral voids and 32 octahedral voids are occupied by metal ions. The dehydration activity of spinel is related to the fraction of B ion in the tetrahedral void. The larger the fraction, the more acidic the surface of the catalyst and the higher the dehydration activity. Generally, the aluminum air battery does not use this catalyst.
(6) Other metals and alloy catalysts. Nickel is relatively inexpensive, and has high corrosion resistance under anodic polarization conditions in an alkaline electrolyte, and nickel has the highest oxygen evolution efficiency among metal elements, so nickel is conventionally used as an alkaline water electrolytic anode material. Alloy catalysts such as nickel iron, nickel cobalt, etc. are often used, which have good catalytic activity and corrosion resistance, and are also a catalyst direction that can be considered for aluminum air batteries.
(7) Composite catalyst. Two or more catalysts are compounded together to better increase the catalytic activity of the air electrode of the aluminum air battery.
Aluminum air battery application prospects
At present, aluminum air batteries have not been widely promoted and applied in industrial and civil fields, mainly due to the need to improve material preparation technology and the concept of secondary charging and discharging.
At the technical level: the measured specific energy and discharge efficiency of the aluminum air battery differs greatly from the theoretical value, and the main technical problems exist, including
(1) The self-corrosion and hydrogen evolution of aluminum anodes restrict the discharge efficiency to a large extent, and the surface passivation of aluminum anodes affects the discharge response aging;
(2) The matching between the electrolyte and the anode can not only form a rapid anodizing reaction response mechanism with the aluminum electrode, but also maintain the efficiency and stability of ion transport, and the cyclability of the oxidation product;
(3) The structure of the air electrode, the self-loss of the current of the current collector layer, and the oxygen reduction ability of the air electrode catalyst are to be further optimized and improved.
The concept of aluminum air battery as a secondary battery: aluminum air battery as a metal fuel battery, the general concept is considered to be a primary battery, which is a misunderstanding of the charge and discharge cycle unit. As a classic secondary battery, the lithium-ion battery that we commonly use now can realize instant charge-discharge conversion. If the aluminum-air battery can realize the industrial charging and discharging process, it can be regarded as two from this large circulation system. Secondary battery, and this is one of the key technologies to solve its promotion and application.
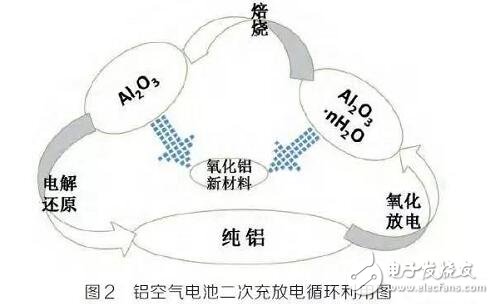
Pure aluminum or alloy undergoes anodization (discharge) to form Al2O3·nH2O, Al2O3·nH2O is calcined to form Al2O3, and further electrolytic reduction (charging) can regenerate aluminum; Al2O3 and
Al2O3·nH2O can also be used as a raw material for the preparation of chemical alumina.
The high specific energy of the aluminum air battery and its safety and environmentally friendly characteristics determine that it will have a broad development prospect, and it will be first applied to mobile devices such as power vehicles and mining in the future. The aluminum air battery can be designed as an integrated battery pack. It can be stored in a gas station or a special charging station like a fuel such as gasoline. During the use, the battery pack can be directly replaced after the anode is consumed. The discharged battery pack is handed over to the separation and recovery of Al2O3·nH2O and the secondary assembly of the battery pack by a professional technology company.
Vape pod system is made up of mini pod and rechargeable vape device, it is designed as an ultra-compact and easy to use vape device. The pod usually use ceramic coil, deliver a more delicate and smooth taste. Moreover, the disposable pod has more flavors, fruit, tobacco, nut etc...let you taste more incredible flavor with great Nic satisfaction. The pod systems are ideal for users who want to make the transition from smoking to vaping or for sophisticated vapers who want a portable device for use on the go.
Pod systems are mostly the mouth-to-lung vaping style. Vape pods nowadays adopt draw-activated mechanism which need no button to operate, take a puff on the device, activate the device immediately.
The advantages of vape pods you should know:
Affordable: the pods are cheaper than Disposable Vapes
Portable and convenient: pod systems are small and easy to carry around.
Discreet: pod systems have minimal vapor production.
Leak-proof: pod systems come with pre-built and disposable cartridges with low chances of leaking.
Stronger nicotine hit: compatible with high nicotine salt-juice and they will give you the satisfying nicotine hit quickly.
2ml e juice capacity
Vape Pod,One Use Vape,Vape Pods Flavor,Vape Shop,Electric Cigarette Vape
Shenzhen Axiswell Technology Co., Ltd , https://www.vapingera.com
